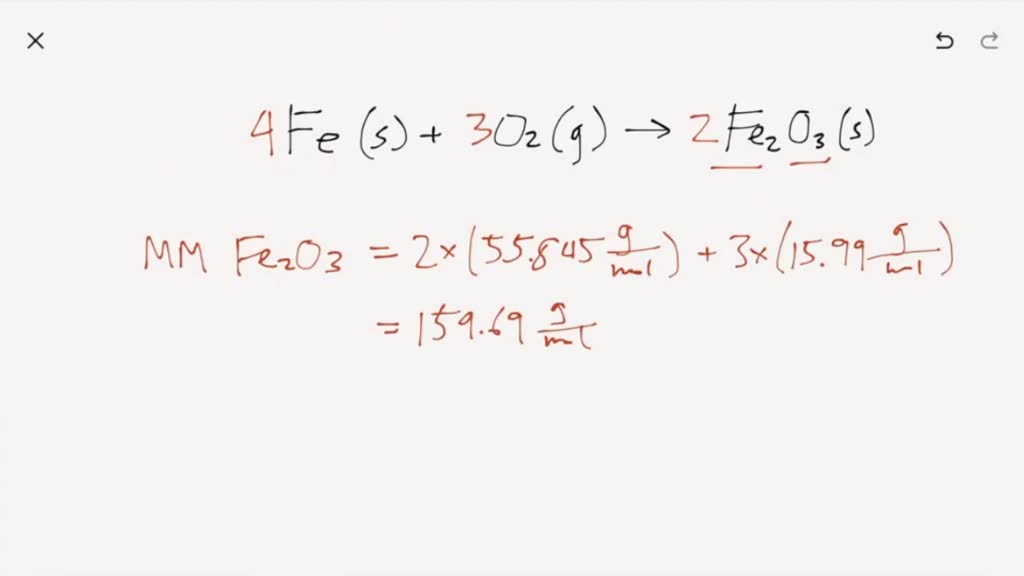SOLVED: Please help with this problem. Oe490 (80 Y L 00 8904 Empirical formula FeO. 2. A 0.450-gram sample of iron oxide was reduced to 0.315 gram of iron metal by reacting

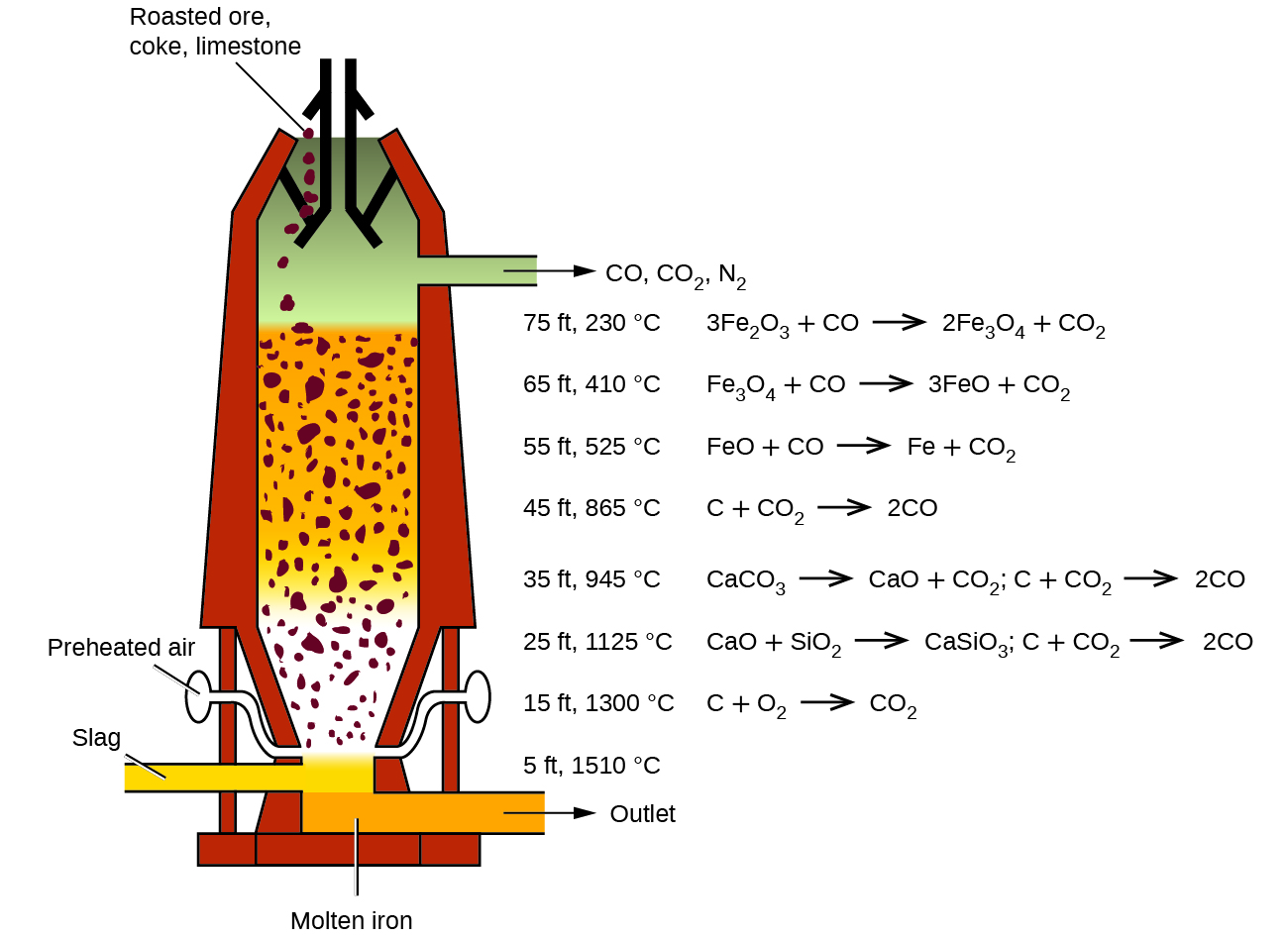
SOLVED: Iron is used to make steel. The two most common iron ores are magnetite and hematite. The chemical process used for smelting is given in the UNBALANCED equation below: If a